The Problem: Disconnected Data and Delayed Decisions
Clinical trial data today is typically scattered across a patchwork of systems. Sponsors might use one platform for EDC, others for ePRO/eCOA, labs, or imaging, and then downstream tools to aggregate and clean the data. This slows everything down: data arrives out of sync, cleaning cycles require multiple “round trips” back to the source, and—crucially—sponsors lack timely oversight of study conduct and patient safety.
The impact on clinical trial execution is significant:
- Lagging Data and Delayed Queries: By the time information reaches a downstream tool it is already stale, and corrections require costly back-and-forth with the source system.
- No Full Lifecycle View: Sponsors cannot easily see the overall state of patients, sites, or the trial—such as query history, SDV progress, or unresolved issues.
- Disjointed Query Management: Queries rarely reach the originator directly; data managers must rely on exports, emails, or separate EDC queries, slowing resolution.
- No Cross-Patient Context: Traditional EDC handles within-patient checks, but anomalies across patients, visits, or data sources often go unseen.
The consequence: slower decisions, higher costs, and longer timelines to deliver results to patients.
Unified Data Capture and Review: A Game Changer
Now imagine a unified platform where capture and cleaning happen together in real time. Instead of shuffling between an EDC and downstream warehouses or listings, all stakeholders work from a single source of truth. Data from sites, patients, labs, and other sources flows into one environment as soon as it is captured.
The advantages are immediate:
- Real-Time Access: Everyone sees the current state of the data without waiting for batch transfers or manual reconciliations.
- End-to-End Oversight: A single global database provides transparency on queries, cleaning status, and study progress.
- Integrated Query Management: Queries can be raised in context and sent directly to the originator—replacing the “download to Excel, send emails” cycle with instant feedback.
- Modern Architecture: Built on single-instance, multi-tenant cloud, these platforms scale to high data volumes and support all major trial functions—EDC, eCOA, RTSM, eConsent, reminders, even document management—under one roof.
The consequence: faster setup, fewer systems to manage, and data issues resolved in hours instead of weeks.
Connector / Plugin Ecosystem
A unified platform does not have to be a closed, monolithic product. In fact, it shouldn’t be. No single vendor can cover every functional demand in clinical research.
The right approach is an open ecosystem:
- Core functionality provided by the platform itself.
- Extension modules that can be built directly on top, sharing the same user interface, security model, and database.
- Open interfaces that let third-party products exchange data bi-directionally, extending the platform’s reach.
This combination of native capability and openness creates a flexible environment. Sponsors get the stability of a central platform with the innovation of an app store — the best of both worlds.
Multi-Dimensional Data Views Across Patients
One of the biggest advantages of an integrated platform is the ability to review data not just within a patient, but across the entire study. Stakeholders with the right permissions can step out of the “one patient at a time” silo and see aggregate patterns and outliers across all participants. This changes how medical monitoring and data quality oversight are done.
Take a pediatric growth study. Traditional EDC might confirm whether each infant’s data falls within expected ranges for age and gender. An integrated platform goes further: a medical monitor can pull up a scatter plot of growth measurements across all patients, plotted against age and external standards such as WHO curves.
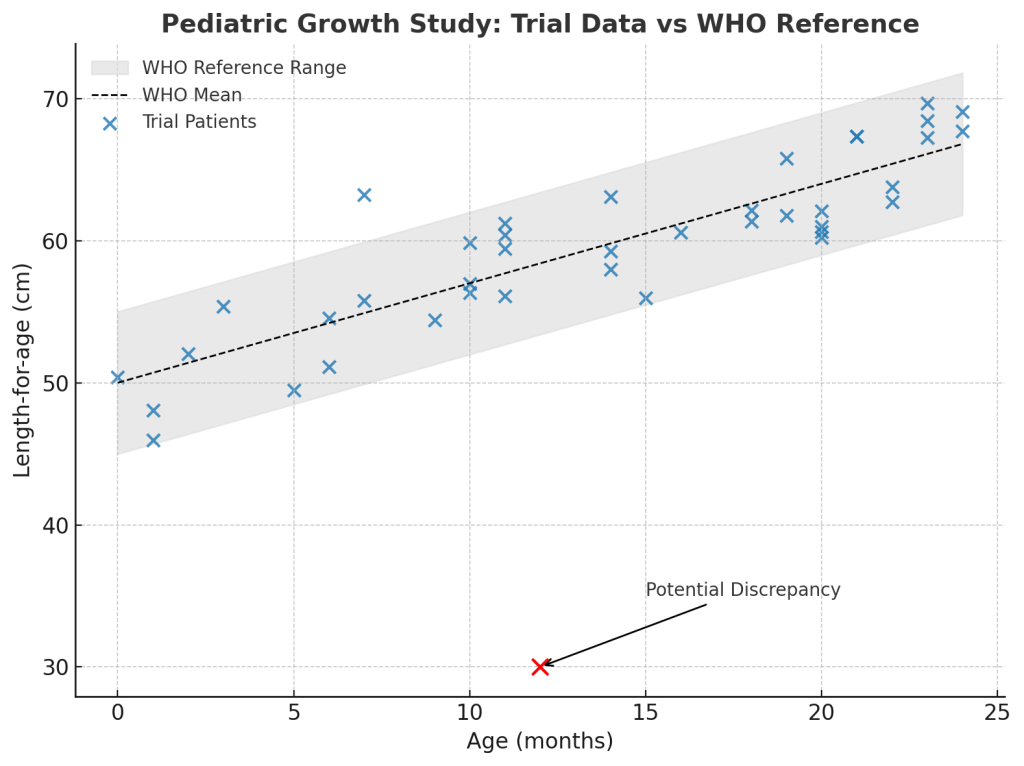
Suddenly, a data point that looked acceptable in isolation stands out as a clear outlier. With a click, the monitor can drill into that patient’s record, view longitudinal data, or compare with peers at similar timepoints. All of this happens inside the platform, in seconds, without exporting data or running separate listings.
This contextual, cross-patient view transforms data cleaning. Anomalies that only reveal themselves longitudinally or in aggregate can be spotted and acted on immediately. Queries or internal notes can be raised directly on the graph, keeping review and resolution in context.
The result: medical monitors and data managers can separate true discrepancies from natural variability far more effectively — patterns that once hid in spreadsheets now surface instantly.
Collaboration in a Multi-Stakeholder Environment
Clinical trials are a team effort. Site staff, data managers, CRAs, medical monitors, project managers, and statisticians all play a role — but too often they work from different systems and outdated views of the data. A unified environment changes that, creating a shared workspace where each role has its own view and tasks, but everyone draws from the same, real-time information.
- Immediate Query Resolution: Discrepancies can be queried at the source. If a site enters an implausible value, the query appears instantly for the site user to resolve — no exports, emails, or delays.
- Unified Task Management: Every user sees their to-do list in one place. As soon as a task is completed, it’s visible to others. CRAs can track query status in real time, avoiding duplication. Compare this with today’s reality, where EDC, ePRO, and RTSM each run their own “task” and “query” systems in isolation.
- Full Visibility: Dashboards show the true state of the trial: open queries, missing forms, deviations, or lagging sites. Issues can be prioritised and tackled early, rather than discovered too late.
- Cross-Functional Insights: Different roles — monitors, data managers, safety teams — all work from the same current dataset. That means meetings start with shared facts, not conflicting reports.
The impact is simple: decisions are faster and more confident because every stakeholder is aligned on the same live data.
AI-Powered Continuous Data Surveillance
An integrated platform opens the door to artificial intelligence (AI) working alongside human reviewers. With data centralised and updated in near real time, an AI agent can scan every new entry for patterns or anomalies — effectively a virtual reviewer on duty 24/7.
Imagine an AI trained on historical trials, therapeutic knowledge, and the specifics of your protocol. The moment a new lab result or diary entry is submitted, it can assess it in the context of the full dataset across patients and visits. In our pediatric growth example, it might flag an infant’s weekly rate as deviating not only from WHO norms but also from peers in the study, and prompt the medical monitor to investigate.
The difference is timing. Instead of waiting weeks for aggregated listings or interim analyses, issues are raised immediately. Data managers and medical monitors can validate the alert, query the site, or take safety action within hours, not weeks.
This is more than convenience. Integration plus AI turns data review into a proactive cycle: errors caught earlier, workloads reduced, safety issues surfaced sooner. In practice, an AI-augmented platform can highlight abnormal patterns, suggest likely entry errors, or even predict patient dropout risk — while leaving final judgement in the hands of human experts.
The impact: fewer blind spots, faster reactions, and a continuous safety net that protects both data integrity and patients.
Performance and Cost Optimisation
Bringing data review into the same platform as data capture isn’t just tidier — it directly improves trial timelines and costs. With integrated, real-time visibility, teams can act faster and avoid the waste that comes from juggling multiple systems.
- Faster Decisions: Clean data is available immediately for interim analyses or adaptations. Sponsors can decide to expand or halt a trial arm based on up-to-date signals, not stale listings.
- Quicker Database Lock: Continuous cleaning throughout the study means the database can be locked within days of last-patient-last-visit, instead of weeks.
- Lower Operational Cost: One platform replaces five. Fewer integrations, fewer reconciliations, and a single training curve all reduce overhead.
- Better Use of Resources: Data managers and monitors spend less time chasing down discrepancies and more time on analysis that matters. Risk-based approaches focus attention where it’s needed most.
- Built to Scale: Modern single-instance platforms handle data from wearables, EHR, and decentralised trials without grinding to a halt — a leap forward from older architectures.
The result: leaner operations, shorter timelines, and freed-up budget to invest where it matters most — advancing the science.
Faster Trials, Better Patient Outcomes
The ultimate benefit of an integrated review platform is speed — not for its own sake, but for patients. Streamlined capture and cleaning shorten development timelines. Effective therapies reach the market faster; ineffective or unsafe ones are identified sooner.
Continuous monitoring means signals don’t sit buried in listings. A study can stop early for success, bringing a treatment to patients months sooner. Or it can stop for futility, sparing participants unnecessary exposure and redirecting resources to better options.
Higher-quality, real-time data also strengthens regulatory submissions. Fewer queries and cleaner datasets reduce the risk of approval delays. At the same time, patients and sites benefit from a simpler trial experience — fewer logins, built-in reminders, seamless eConsent and ePRO. That improves compliance, completeness, and ultimately the evidence base.
The impact is clear: faster decisions, better data, and earlier access to safe and effective therapies for patients.
Conclusion: The Future of Integrated Data Review
The way we manage trial data is changing. The days of siloed systems and late reconciliations are numbered. A connected, real-time platform – where data entry, querying, and review happen in the same environment – removes the lag, reduces cost, and gives sponsors the oversight they need to make faster, better decisions.
This isn’t science fiction. These platforms already exist. They cover the full range of trial functions – EDC, eCOA, RTSM, eConsent, reminders – in a single, task-driven environment. And because they are built on modern, single-instance, multi-tenant architecture, they are built to scale.
The opportunity is clear. Tear down the silos, shorten the cycle, and give stakeholders the ability to act on data as it arrives. The result is faster decisions, lower cost, and – most importantly – quicker access to the right therapies for patients.
Discover more from ClinFlo Consulting
Subscribe to get the latest posts sent to your email.